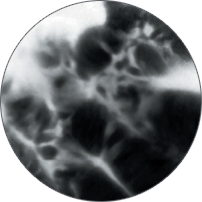
Cellvizio® vision is changing lives
Cellvizio, the world’s only real-time in vivo cellular imaging platform, is transforming gastroenterology. By enabling you to see precisely what’s occurring virtually anywhere in the GI tract, at the cellular level, in real time, Cellvizio reduces uncertainty and delay in the diagnosis and treatment of gastrointestinal diseases:1
Obtain instant relevant diagnostic information2,3
Make on-the-spot treatment decisions1
Evaluate response to treatment quickly and precisely4
The Cellvizio® difference
Early, accurate diagnosis of gastrointestinal disease can be difficult with traditional endoscopic techniques, which offer limited diagnostic and therapeutic capabilities. The power of Cellvizio vision changes that.

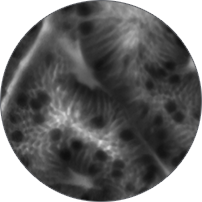
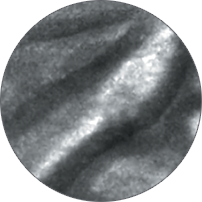
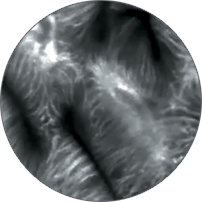
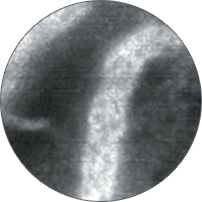
Instead of blind biopsies, view the characteristics of cells in real time, inside the patient, with high-magnification video. Observe the mucosa for investigation of potentially cancerous tissue.

Classify high-grade dysplasia and early esophageal cancer with high accuracy.5 Characterize intestinal metaplasia and early gastric cancer.6 Differentiate responders from non-responders for ulcerative colitis patients.4

Perform better targeted biopsies,5 yielding on-the-spot characterization of abnormalities. Identify cancers,7 or rule out malignancies with more confidence,8 and avoid biopsies of insignificant lesions.9

Make earlier, more conclusive diagnoses.10 Perform fewer, less invasive interventions.11 Optimize treatment paths and patient care, for more favorable clinical12 and economic outcomes.9

The Cellvizio® platform
The Cellvizio real-time in vivo cellular imaging platform uses Confocal Laser Endomicroscopy (CLE) advanced imaging technology to deliver cellular visualization virtually anywhere in the human body.
Learn more- Wang K. et al. Use of probe-based confocal laser endomicroscopy (pCLE) in gastrointestinal applications. A consensus report based on clinical evidence. UEGW Journal, 2015.
- Ji R, et al. Confocal endomicroscopy for in vivo prediction of completeness after endoscopic mucosal resection. Surg Endosc. 2011;25:1933-1938.
- Jeon SR, et al. Optical biopsies by confocal endomicroscopy prevent additive endoscopic biopsies before endoscopic submucosal dissection in gastric epithelial neoplasias: a prospective, comparative study.Gastrointest Endosc. 2011;74:772-780.
- Hundorfean G. et al. Development and Validation of a Confocal Laser Endomicroscopy-Based Score for In Vivo Assessment of Mucosal Healing in Ulcerative Colitis Patients. Inflamm Dis. 2017.
- Sharma P. et al. Real-time increased detection of Neoplastic tissue in Barrett’s Esophagus with pCLE; Final results of a multi-center prospective international randomized controlled trial, Gastrointest Endosc. 2011.
- Li Zhen et al. Confocal laser endomicroscopy for in vivo detection of gastric intestinal metaplasia: a randomized controlled trial, Endoscopy, 2013.
- Shahid M.W. et al. Diagnostic Accuracy of probe based Confocal Laser Endomicroscopy in Detecting Residual Colorectal Neoplasia after EMR: A prospective Study. Gastrointest Endosc. 2012.
- Kiesslich R. et al. In vivo histology of Barrett’s Esophagus and Associated Neoplasia by CLE, Clinical Gastro and Hepatology, 2006.
- Al-Mansour. et al. SAGES TAVAC safety and efficacy analysis confocal laser endomicroscopy. Endosc. 2020.
- Napoléon B, et al. Confocal endomicroscopy for evaluation of pancreatic cystic lesions: a systematic review and international Delphi consensus report. Endosc Int Open. 2020.
- Le Pen C, et al. A health economic evaluation of needle-based Confocal Laser Endomicroscopy for the diagnosis of pancreatic cysts. Endosc Int Open. 2017.
- Palazzo et al. Impact of needle-based confocal laser endomicroscopy on the therapeutic management of single pancreatic cystic lesions, Surg Endosc. 2019.
Cellvizio® I.V.E. with Confocal MiniprobesTM are regulated Medical Devices, CE marked (CE 0459) (Class IIa - NB :G-MED) and FDA cleared. Cellvizio® is a registered trademark and Confocal MiniprobeTM is a trademark of Mauna Kea Technologies. Cellvizio® I.V.E. with Confocal MiniprobesTM is a confocal laser system with fiber optic probes that are intended to allow imaging of the internal microstructure of tissues including, but not limited to, the identification of cells and vessels and their organization or architecture. These statements and the associated reference to specific clinical studies, are not intended to represent claims of safety or effectiveness for detecting or treating any specific condition or disease state. Rather this information is intended to provide useful reference to selected published literature describing physician experiences with the associated clinical uses. Any diagnostic assessment should always be made by the attending physician, based on the evaluation of all sources of clinical, endoscopic and other relevant information. These statements have not been reviewed, cleared, or approved by the U.S. FDA. The use of this medical device is exclusively reserved for healthcare professionals