Pancreatic Cysts
Cellvizio® is the real-time in vivo cellular imaging platform that significantly improves the classification of indeterminate cysts quickly and with more certainty.
Up to 30% of cases remain indeterminate after endoscopic ultrasonography (EUS)1 and over 50% of cysts are inconclusive after fine-needle aspiration (FNA).2
As a result, 60% of patients with benign pancreatic cysts undergo unnecessary surgery due to uncertain diagnoses.3 Moreover, standard of care accuracy for the diagnosis of mucinous pancreatic cystic lesions (PCLs) is 71%.4
Cellvizio improves diagnostic accuracy, enabling you to confidently manage patients and reduce surgical intervention.
Cellvizio® Clinical Value
Cellvizio® improves characterization of indeterminate cysts5, which allows physicians to reduce surgical intervention by 23%.6 As a result, 35% of patients with benign serous cystadenoma (SCA) are prevented from further surveillance.5
Needle-based Confocal Laser Endomicroscopy (nCLE) with Cellvizio® allows you to see the cyst wall at the microscopic level, in real time, for the first time through a high-speed flexible microscope threaded inside a 19-gauge needle during EUS-FNA procedures. This improves accuracy for the diagnosis of mucinous PCLs to 97% with nCLE alone.4
See one patient case of nCLE in Pancreatic Cyst Assessment with Dr. Bertrand Napoléon, a gastroenterologist and interventional endoscopist.
“My approach to evaluating pancreatic cystic lesions is revolutionized, creating a major change in patient management."
-- Dr. C. J. DiMaio, Mount Sinai Hospital
Patient Management
With Cellvizio, diagnosis is more conclusive. The outcomes are positive results that help to completely change patient management by characterizing indeterminate cysts. Therapeutic decisions are modified by 28%, which eliminates follow ups for some patients. Surveillance of benign SCA is eliminated in 35% of cases.5 This allows for a 23% reduction in unnecessary surgeries and a significant economic benefit of a 13% reduction in cost.6
The benefits of Cellvizio have further been underscored most recently in a meta-analysis comprising 3,641 patients with cysts, finding that adding Cellvizio to an EUS procedure was a better choice for the diagnosis of PCLs for centers with relevant expertise and facilities.8

Economic Benefit
In addition to the proven clinical and patient management benefits, Cellvizio also offers downstream revenue opportunities by accurate upstaging and downstaging of PCLs. A study adding nCLE to EUS-FNA procedures showed potential cost-benefits in the management of PCLs (≥ 3 cm) by preventing at least one unnecessary pancreatic surgery for every ten subjects undergoing evaluation by current practices.7
Cellvizio advanced imaging enables you to select the right patients for surgery and for follow-up, directing your time and your facility's resources more efficiently.
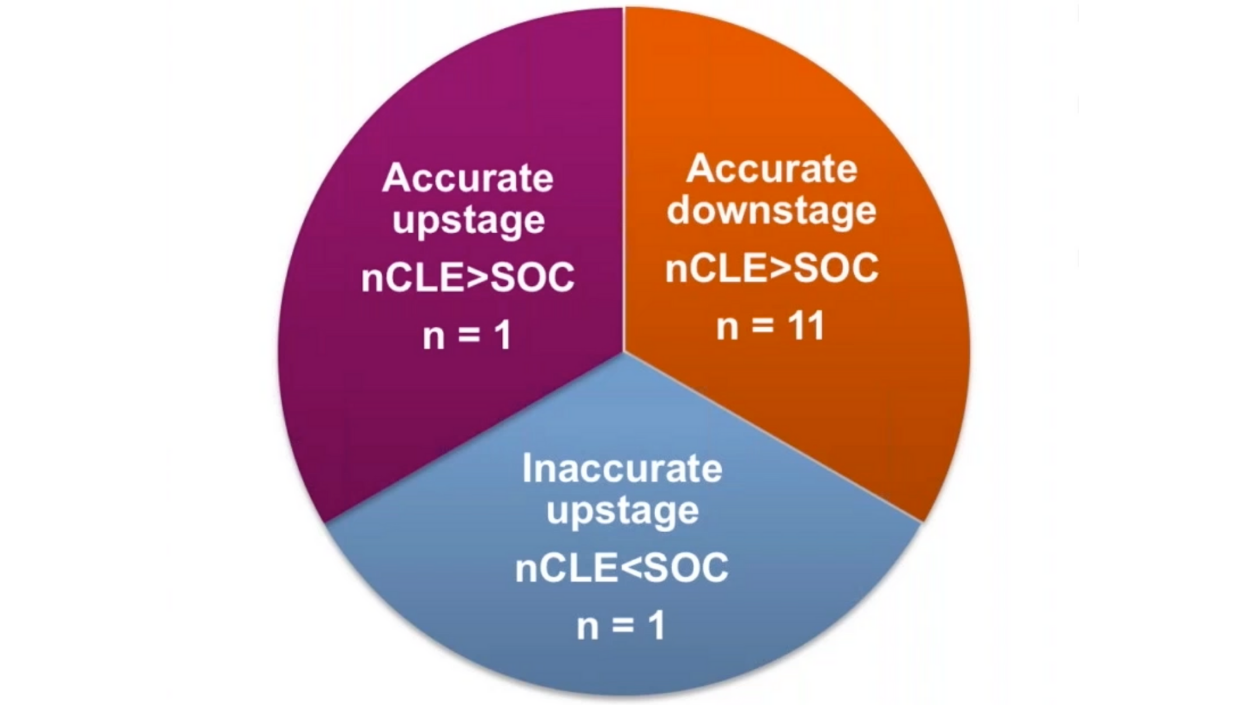
Learn more about the cost-benefit analysis here.
Characterization of Cyst
using Cellvizio® AQ-FlexTM 19 Miniprobe
Non-Mucinous - Serous cystadenoma
Criteria: superficial vascular networking
Mucinous IPMN
Criteria: papillary projection
Mucinous cystadenoma
Criteria: epithelial border
1. Rodríguez-D’Jesús A, et al. Impact of endoscopic ultrasonography (EUS) and EUS-guided fine-needle aspiration on the management of pancreatic cystic lesions. Eur J Gastroenterol Hepatol, 2016.
2. Thornton GD. et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology, 2013.
3. Jais B. et al. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut 2015
4. Krishna SG, et al. Endoscopic Ultrasound-Guided Confocal Laser Endomicroscopy Increases Accuracy of Differentiation of Pancreatic Cystic Lesions. Clinical Gastroenterology and Hepatology, 2019.
5. Palazzo et al. Impact of needle-based confocal laser endomicroscopy on the therapeutic management of single pancreatic cystic lesions, Surgical Endoscopy, 2019.
6. Le Pen C et al. A health economic evaluation of needle-based Confocal Laser Endomicroscopy for the diagnosis of pancreatic cysts. Endoscopy International Open (2017).
7. Luthra A, et al. Cost-Benefit Analysis and Resource Implications of Endoscopy Ultrasound-guided Confocal Endomicroscopy in Pancreas Cysts, Techniques and Innovations in Gastrointestinal Endoscopy, 2021. https://doi.org/10.1016/j.tige.2021.10.002.
8. Li SY, et al. Comparative Performance of Endoscopic Ultrasound-Based Techniques in Patients With Pancreatic Cystic Lesions: A Network Meta- Analysis. The American Journal of Gastroenterology, 2023.
Cellvizio® I.V.E. with Confocal MiniprobesTM are regulated Medical Devices, CE marked (CE 0459) (Class IIa - NB : G-MED) and FDA cleared. Cellvizio® is a registered trademark and Confocal MiniprobeTM is a trademark of Mauna Kea Technologies. Cellvizio® I.V.E. with Confocal MiniprobesTM is a confocal laser system with fiber optic probes that are intended to allow imaging of the internal microstructure of tissues including, but not limited to, the identification of cells and vessels and their organization or architecture. FDA clearance: indications for use: Once connected to the Cellvizio® I.V.E: The AQ-FlexTM 19 Confocal MiniprobesTM are intended to allow imaging of anatomical tracts, i.e., gastrointestinal and respiratory tracts, accessed by an endoscope, or endoscopic accessories (e.g. aspiration needles used during procedures including EUS-FNA, EBUS-TBNA and TBNA needles). CE marked: indications for use: Once connected to the Cellvizio® I.V.E: The AQ-FlexTM 19 Confocal MiniprobesTM are intended to allow imaging of anatomical tracts, i.e., gastrointestinal tracts and respiratory tracts accessed by an endoscope or endoscopic accessories, including through endoscopic needles. Please consult labels and instructions for use. These statements and the associated reference to specific clinical studies, are not intended to represent claims of safety or effectiveness for detecting or treating any specific condition or disease state. Rather this information is intended to provide useful reference to selected published literature describing physician experiences with the associated clinical uses. Any diagnostic assessment should always be made by the attending physician, based on the evaluation of all sources of clinical, endoscopic and other relevant information. These statements have not been reviewed, cleared, or approved by the U.S. FDA. The use of this medical device is exclusively reserved for health professionals. Product availability cannot be guaranteed in all countries. For further information, please contact your local sales
representative.